Introduction
As shown in previous blogs (MOSH/MOAH), the GERSTEL MPS smart series robotic sampler enables the automated separation and determination of MOSH and MOAH, food safety contaminants found in edible oils (AppNote 228, AppBriefs 19 and 20). This blog will discuss how the same robotic sampler can also be used to automate the extraction and determination of the 3-MCPD, 2-MCPD, and glycidol esters, process contaminants formed when edible oils and fats are refined at high temperatures during their deodorization process.
Understanding the Contaminants
3-Monochloropropanediol (3-MCPD), 2-Monochloropropanediol (2-MCPD), and glycidol esters are process contaminants that form when edible oils and fats are refined at high temperatures in the presence of chloride ions.
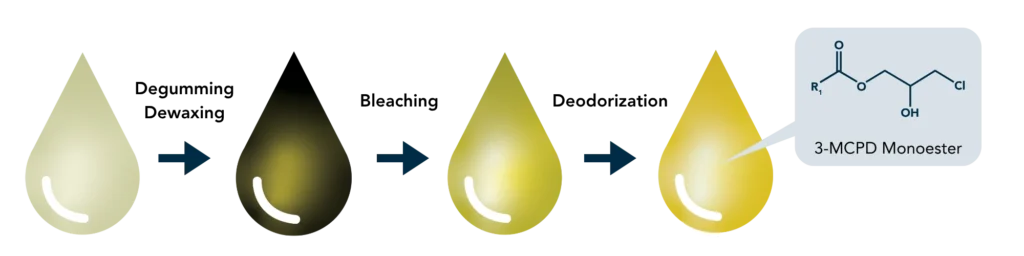
Once ingested, these esters can hydrolyze into their free forms—3-MCPD, 2-MCPD, and glycidol—compounds that are toxicologically concerning. Glycidol is classified by the International Agency for Research on Cancer (IARC) as probably carcinogenic to humans (Group 2A), while 3-MCPD is considered possibly carcinogenic (Group 2B). Consequently, the European Union has set strict maximum limits for their presence in edible oils and fatty foods, particularly in infant formulas and baby food, where levels must often remain below 10–50 µg/kg.
Standardized Methods for Determination
Several standardized methods exist for determining ester-bound MCPDs and glycidol in fats and oils. The key methods—ISO 18363-1, ISO 18363-2, ISO 18363-3, and ISO 18363-4—all rely on transesterification to release the bound analytes, derivatization with phenylboronic acid (PBA), and subsequent analysis by gas chromatography coupled with mass spectrometry (GC-MS or GC-MS/MS).
- ISO 18363-1 (AOCS Cd 29c-13): The most widely used “C-method,” employing a fast alkaline transesterification followed by differential calculation of glycidol. While rapid, this method tends to overestimate glycidol in samples with high 3-MCPD.
- ISO 18363-2 and -3: Acidic or slow-alkaline variants recognized for their high precision but requiring long incubation times (up to 16 h) and manual handling—better suited to reference laboratories.
- ISO 18363-4 (Zwagerman/Overman Method): The most recent addition (2021), designed for direct quantification of 3-MCPD, 2-MCPD, and glycidol in a single assay. It introduces a ¹³C-label correction step that compensates for glycidol overestimation caused by 3-MCPD degradation during transesterification. This makes it the most robust and scientifically advanced approach currently standardized for routine food-safety monitoring.

Automating the ISO 18363-4 Workflow
GERSTEL has demonstrated that the entire ISO 18363-4 workflow can be fully automated using the MultiPurpose Sampler (MPS) equipped with modules for mixing, cooling, agitation, solvent addition, and sample transfer.
In the automated sequence, the analyst only needs to weigh an aliquot of oil into a sample vial and place it onto the MPS.
All subsequent operations, including:
- addition of solvents and reagents,
- transesterification,
- quenching,
- extraction,
- derivatization,
- and GC-MS/MS injection—
are executed automatically under control of GERSTEL’s MAESTRO software.

The method achieves limits of quantification well below 20 µg/kg for all three analytes—far surpassing the 100 µg/kg thresholds defined in the ISO standard. Precision values are typically below 6 % RSD, and recoveries between 90 and 110 %, even for complex matrices such as frying oils or vegetable blends.
Using a pre-column backflush system, the MPS-GC-MS/MS setup maintains clean analytical columns and mass spectrometers, minimizes maintenance, and enables continuous 24-hour processing of up to 45 samples per day.
Why Automation Matters in Food Safety Testing
Automating the determination of process contaminants such as 3-MCPD, 2-MCPD, and glycidol esters offers significant advantages:
Consistency and Accuracy: Robotic precision minimizes human error in pipetting, timing, and temperature control—critical for reliable quantification at trace levels.
High Throughput: Automated workflows enable overnight and unattended operation, dramatically increasing sample capacity and supporting regulatory compliance in production and contract laboratories.
Method Robustness: Integrated backflushing, temperature-controlled modules, and standardized reagent handling reduce contamination and instrument downtime.
Regulatory Confidence: Fully automated methods that reproduce ISO 18363-4 conditions ensure that results are defensible in audits and interlaboratory comparisons.
Conclusion
The GERSTEL MultiPurpose Sampler (MPS), when configured for the ISO 18363-4 Zwagerman/Overman method, delivers a comprehensive automated solution for the determination of 3-MCPD, 2-MCPD, and glycidol esters in edible oils and fats. This integration of standardized methodology with intelligent automation ensures precise, reproducible, and regulation-compliant analysis—helping food safety laboratories meet the growing global demand for accurate contaminant monitoring while maintaining efficiency and data integrity.